Welcome to Guangzhou IGE Biotechnology Co., Ltd. Website !
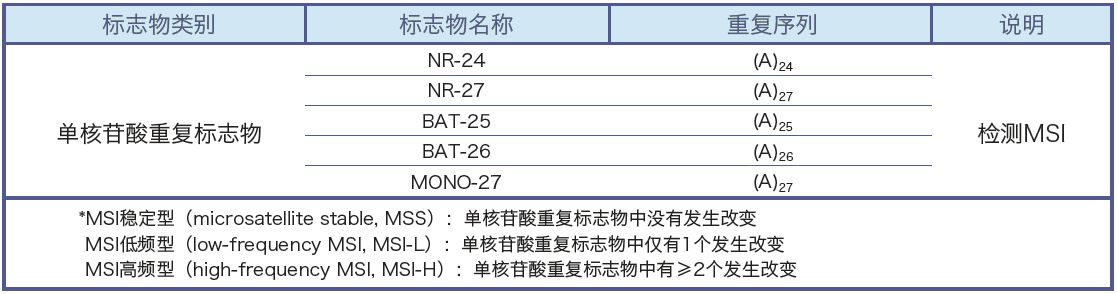
Microsatellites are short, tandemly repeated sequences that are scattered throughout the human genome. In tumor tissue, microsatellites can undergo insertions or deletions of repeat units, resulting in changes in microsatellite length. This phenomenon is called microsatellite instability (MSI).
MSI is caused by defects in mismatch repair (MMR) genes. MMR genes are responsible for correcting errors in DNA replication. When MMR genes are defective, microsatellites are more likely to undergo insertions or deletions.
MSI is an important prognostic factor for colorectal cancer. Patients with MSI-high (MSI-H) colorectal cancer have a worse prognosis than patients with MSI-low (MSI-L) or microsatellite stable (MSS) colorectal cancer. MSI-H colorectal cancer patients are also more likely to respond to immunotherapy drugs such as PD-1/PD-L1 inhibitors.
MSI is also a diagnostic criterion for Lynch syndrome. Lynch syndrome is an autosomal dominant genetic disorder that is characterized by defects in MMR genes. Patients with Lynch syndrome are at increased risk for developing multiple cancers, including colorectal cancer, endometrial cancer, gastric cancer, and pancreatic cancer.
Clinically, MSI testing is used for the following purposes:
To guide the use of PD-1/PD-L1 inhibitors in colorectal cancer patients
To guide the choice of adjuvant treatment regimens in patients with stage II colorectal cancer
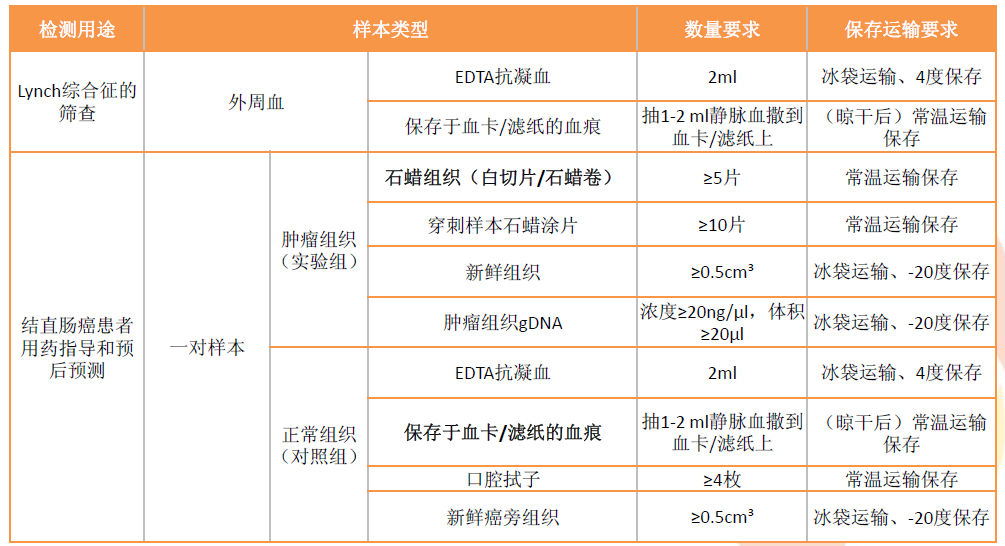
To screen for Lynch syndrome
Gold standard: The gold standard method for MSI testing is to use a first-generation sequencing platform to detect MSI status using an internationally recognized multiplex fluorescent PCR-capillary electrophoresis assay.
Time: The test is a one-tube amplification assay that can be completed within 5 business days of sample receipt by the laboratory.
Content: The test includes all single-nucleotide positions recommended by the National Cancer Institute (NCI), as well as additional positions that can be used to identify potential sample contamination or mix-ups, which can reduce the risk of errors.